how many valence electrons does ge have|Valence Electrons Chart for All Elements : iloilo Ge has 18 valence electrons, according to the table of element valences. The table shows the number of electrons with which an atom will bond or form for each element, based . 対象となる製品のご契約について自動更新するように設定していただいている場合、McAfee から無償の追加特典の適用対象とさせていただくことがあります。これら特典の適用状況は、お客様のマイアカウントページからご確認いただけます。お客様がお住い .
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Germanium (Ge)?
PH3 · Germanium Valence Electrons
PH4 · Germanium Electron Configuration (Ge) with Orbital Diagram
PH5 · Germanium (Ge)
PH6 · Germanium
PH7 · Electron Configuration for Germanium (Ge, Ge2+, Ge4+ ions)
PH8 · Determine valence electrons using the periodic table
PH9 · 10.6: Valence Electrons
Pinay -Hindi kinaya ang laki ng tite ng bf (can't handle the size of dick)-SingCan . Sing Can. 10.4M views. 88%. 54 years ago. 4:15. Pinay GF kong sexy di kinaya ang aking malaking tite (College na wasak) . Hindi kinaya ni Ate Kristine ang Tite ng booking niyang 40 yrs old (Hardfuck/Creampie) Kristine Ramos. 107K views. 84%. 54 years ago. .
how many valence electrons does ge have*******Valence electrons; 1: Hydrogen (H) 1: 2: Helium (He) 2: 3: Lithium (Li) 1: 4: Beryllium (Be) 2: 5: Boron (B) 3: 6: Carbon (C) 4: 7: Nitrogen (N) 5: 8: Oxygen (O) 6: 9: Fluorine (F) 7: 10: Neon (Ne) 8: 11: Sodium (Na) 1: 12: Magnesium (Mg) 2: 13: Aluminum (Al) 3: 14: Silicon .
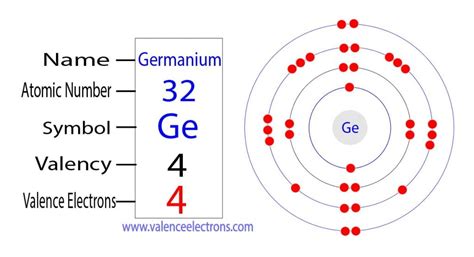
how many valence electrons does ge have Valence Electrons Chart for All Elements The valence electrons of germanium are four, as shown by its electron configuration and the valency of the element. The valence electrons are the total number of electrons in .Valence Electrons Chart for All Elements Ge has 18 valence electrons, according to the table of element valences. The table shows the number of electrons with which an atom will bond or form for each element, based .The atomic number of germanium is 32. That is, the number of electrons in germanium is thirty-two. Therefore, the germanium atom will have two electrons in the first shell, eight .The element has a fixed atomic number of 32. Flerovium Valence Electrons. Helium Valence Electrons. Plutonium Valence Electrons. Lithium Valence Electrons. .
Germanium is a Metalloid element. It is part of group 14 (carbon family). Know everything about Germanium Facts, Physical Properties, Chemical Properties, Electronic .
Germanium. Discovery date. 1886. Discovered by. Clemens Winkler. Origin of the name. The name is derived from the Latin name for Germany, 'Germania'. Allotropes. α-Ge, ß .
A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons .There are four valence electrons in the outer shell of the Germanium. Ground State Electron Configuration of Ge. [Ar].3d 10 .4s 2 .4p 2 is the ground state electron .how many valence electrons does ge haveHow many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence .
For the Ge structure use the periodic table to find the total number of valence electrons for the Ge molecule. Once we know how many valence electrons there .

Chemistry. Chemistry questions and answers. How many valence electrons does Ge (germanium) have? A. 9 B. 8. c. 7 F. 4 G. 3. H 2 D. 6 II E. 5 J. O Draw the correct Lewis-Dot Structure for the PO; (phosphite) .Give the electron configuration for gallium. How many valence electrons does it have? How many valence electrons are in the Lewis-dot (electron dot) structure for the neutral silicon (Si) atom? An atom of germanium (Ge) has a diameter of about 1.3 times 10^{-8} cm. (a) What is the radius of a germanium atom in angstroms?
Step-2: Need to do electron configuration of beryllium. Step 2 is very important. In this step, the electrons of beryllium have to be arranged. We know that beryllium atoms have a total of four electrons. The electron configuration of beryllium shows that there are two electrons in the K shell and two in the L shell. And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system .
Look at the electron configuration for gallium (Ga). It has 3 valence electrons and 28 core electrons. core valence electrons ge Ge core electrons Ge electron configuration As valence electrons. For the main group representative elements, the valence electrons are the outermost highest energy #s#and#p#electrons, which make up the .
The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons. Transition elements or transition metals.Comprehensive information for the element Germanium - Ge is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Valence Electrons: 4s 2 p 2 Electron Dot Model. Chemical Properties of Germanium. Electrochemical Equivalent: . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Each has four valence electrons, but germanium will at a given temperature have more free electrons and a higher conductivity. Silicon is by far the more widely used semiconductor for electronics, partly because it can be used at much higher temperatures than germanium. Silicon crystal structure. Discussion of lattice.How many valence electrons does Ge have? d. What is the net charge of the atomic core for Ge? (Atomic core = nucleus + core electrons) e. Find a picture of the diamond unit cell (a.k.a. diamond lattice) and sketch how an atom is bonded to its nearest neighbors. Note: Si atoms, Ge atoms, and other semiconductors (including the diamond form of .
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . Valences of the Elements Chemistry Table. You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
The diagram below shows the number of valence electrons (VE) for the main group elements. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = . 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step. Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of Germanium is 32. It is a hard, lustrous, greyish-white metalloid of the carbon group. . How Many Valence Electrons Does Germanium Have. Germanium has four valence electrons in its outer shell. As .Study with Quizlet and memorize flashcards containing terms like How many valence electrons does Ge have?, Joey was taking a chemistry test. In one question, he was .In this case, the germanium ion (Ge2+) has a total of two valence electrons. On the other hand, the germanium atom donates two electrons in the 4p orbital and two electrons in the 4s orbital to convert the germanium ion (Ge4+). Ge – 4e– → Ge4+. Here, the electron configuration of germanium ion (Ge4+) is 1s2 2s2 2p6 3s2 3p6 3d10.
California Beach Pansol has four (4) elegant villas fully-equipped with premium facilities and amenities to make your stay even more special. The highest degree of comfort and convenience awaits .
how many valence electrons does ge have|Valence Electrons Chart for All Elements